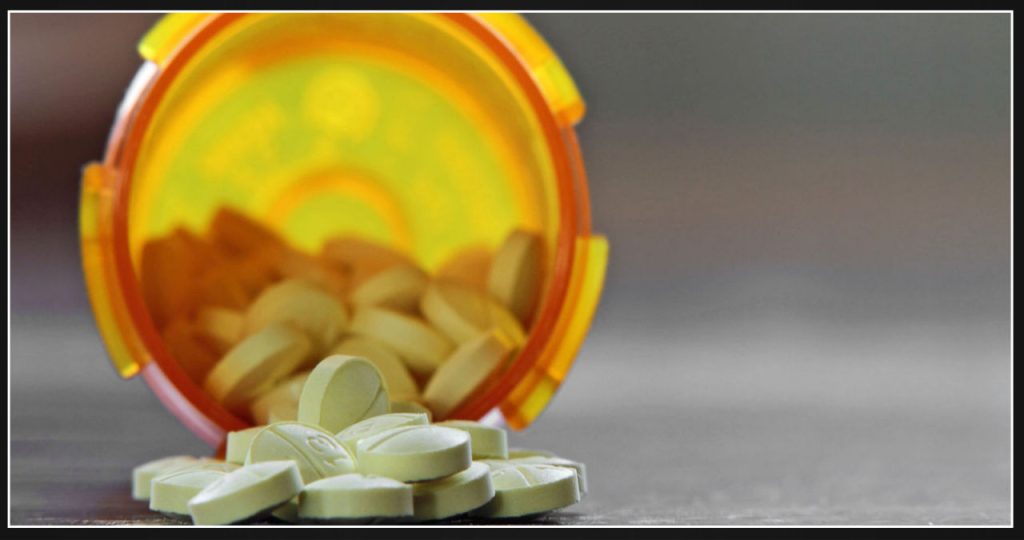
A nationwide recall has been issued for a popular anxiety drug due to a potentially life-threatening error on its carton. Endo, the drug maker, has announced the recall for its prescription-based clonazepam tablets.
Endo is expanding its recall due to the presence of both incorrect drug code and drug strength in some cartons.
Endo recently issued a statement regarding the investigation they are currently conducting. The investigation has revealed a potential issue with certain batches of Clonazepam. It has been discovered that a limited number of cartons were printed with the wrong strength and National Drug Code (NDC) code. This error occurred during the packaging process by a third-party packager. However, it is important to note that the blister strips and tablets within the product packs are labeled correctly with the appropriate strength for each specific lot.
Anxiety Medication Recalled
The recall impacted a total of 16 lots of Clonazepam Orally Disintegrating Tablets, USP (C-IV), which are widely used for treating anxiety. The doses of this popular medication varied from 0.125 milligrams to 2 milligrams. Each package consists of 10 blister strips, with each strip containing six tablets.
The expiration dates for the anxiety medication range from August 2026 to February 2027. Endo, the manufacturer, issued a warning regarding the potential risks associated with this widely-used medication. They emphasized that individuals, both children and adults, who unintentionally consume a higher dosage of clonazepam may face an increased risk of experiencing adverse effects such as significant sedation, confusion, dizziness, reduced reflexes, ataxia, and hypotonia. There is a significant likelihood of severe respiratory depression, which could be life-threatening, particularly for patients with existing pulmonary conditions. This risk is amplified for patients who are prescribed doses near the maximum limit, as well as those concurrently taking other medications that may contribute to further respiratory depression.
Clonazepan is commonly prescribed to alleviate symptoms of anxiety disorders, and it can also be utilized as an effective seizure medication. However, it is crucial to be aware of the potential risks associated with taking a high dose, which may occur due to mislabeling. In light of this, the drug manufacturer has provided specific instructions to follow if you are currently taking this medication.
Endo has distributed the product lots through wholesale distributors to retail pharmacies nationwide. They are taking the necessary steps to notify wholesale accounts and retailers about the product lots and arrange for the return of all existing inventory through Inmar, Inc.
The company stated that distributors and retailers who have the recalled product should stop distributing and dispensing it right away and return it to the place of purchase or contact Inmar using the provided telephone line. Consumers who have any unused prescribed tablet cartons of Clonazepam Orally Disintegrating tablets, USP with the specified lot numbers are advised to stop using the product. If a patient accidentally took an incorrect dose instead of the intended dose, they are advised to consult a physician.
Also Read:
- Texas man plead guilty for distributing liquid methamphetamine in South Texas
- Previewing the Texas Legislative Session: Is a Doubling of the State Minimum Wage Possible?